Auditory Physiology Lab

Overview
The mission of the USF Health Auditory Physiology Laboratory is to improve the application of clinical otology and audiology through advanced research of the ear.
With this mission in mind, our laboratory leverage animal models in addition to human participants. Our laboratory is led by Principal Investigator Jeffery T. Lichtenhan, PhD, who is an experienced researcher that works to identify the origins of physiologic measurements from normal and diseased ears. Physiologic measurements from the ear are routinely used to assess and study hearing in the clinic and in the laboratory. However, these measurements are limited because little has been discovered about their cellular origins and contributing mechanisms.
Our research uses innovative approaches to address questions related to the origins of objective measurements of hearing in normal and diseased ears.
Discoveries and Innovations
Understanding the mechanisms of low-frequency hearing and hearing loss is important because speech sounds, music, and bothersome environmental background noises are primarily low frequency content. Unfortunately, conventional objective measures such as the auditory brainstem response do not adequately assess low frequency hearing. To address this gap, we developed a new objective measurement of low frequency hearing, the Auditory Nerve Overlapped Waveform (ANOW). We found that ANOW thresholds relate to low-characteristic frequency single-auditory nerve fiber (ANF) thresholds in cat just as well as classical auditory nerve compound action potential (CAP) thresholds relate to high-characteristic frequency single ANF thresholds (Lichtenhan et al. 2013). We further elucidated this relationship by showing that i) ANOW originates from afferent ANF in the apical half of the cochlear spiral, ii) ANOW does not originate from phase locking in low-frequency tails of high-characteristic frequency ANF, and iii) measurements from high signal levels are not ANOW and are instead the Auditory Nerve Neurophonic that has unknown cellular or spatial origins along the cochlear length (Lichtenhan et al. 2014). Development of the ANOW technique empowers efforts to overcome barriers of high clinical and scientific interest, such as accurately assessing low frequency hearing in newborn hearing testing and understanding the origin of low frequency hearing loss in animal models of Ménière’s disease.
- Lichtenhan JT, Cooper NP, Guinan JJ Jr. A new auditory threshold estimation technique for low frequencies: Proof of concept. Ear and Hearing. 2013. 34(1):42-51. PMID: 22874644; PMCID: PMC3495092.
- Lichtenhan JT, Hartsock JJ, Gill RM, Guinan JJ, Salt AN. The auditory nerve overlapped waveform (ANOW) originates in the cochlear apex. Journal of the Association for Research in Otolaryngology. 2014. 15(3):395-411. PMID: 24515339; PMCID: PMC4010591.
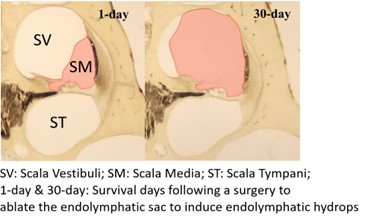
Endolymphatic hydrops is linked to Ménière’s disease, a common disorder of the inner ear. The symptoms of Ménière’s disease are some of the most debilitating of all inner ear disorders and include vertigo, tinnitus, sensations of aural fullness, and low-frequency sensorineural hearing loss. Most clinical treatments for Ménière’s disease that are designed to preserve hearing and vestibular function do little to alleviate the symptoms long term. One possible reason for this shortcoming is that treatment often does not begin until the ear is already permanently damaged. We hypothesize that if the condition could be identified earlier, some of the treatments that should work in theory, but do not in clinical practice, could prevent permanent damage and help alleviate symptoms. We recently induced small volumes of artificial endolymph into the guinea pig cochlea to simulate endolymphatic hydrops and found that Auditory Nerve Overlapped Waveform (ANOW) measurements of low-frequency hearing detected changes while traditional, high-frequency objective measurements of hearing were unaffected (Lichtenhan et al. 2017). This suggests that excess endolymph collects in the distensible cochlear apex, and that low-frequency hearing measurements could be used to more rapidly detect dysfunction associated with the development of endolymphatic hydrops. But, since humans present to the clinic with chronic conditions, and not acute, we developed a chronic guinea pig model of endolymphatic hydrops (Valenzuela et al. 2020a). We found that, indeed, chronic low-frequency hearing dysfunction preceded the development of histologically measurable endolymphatic hydrops (Lee et al. 2020).
In the later stages of the condition, when endolymphatic hydrops is permanent, the approaches used in our laboratory can address a longstanding question in our field: What is the origin of the low-frequency hearing loss in ears with Ménière’s disease? If the origin of the hearing loss were known, current therapies could be improved or new therapies could be developed. Histological measures from human temporal bones from patients with Ménière’s disease, as well as our guinea pig model (Valenzuela et al. 2020b), do not have sensory cell loss at low-frequency regions, meaning that the origin of the low-frequency loss is still a mystery. One hypothesis is that the low-frequency hearing loss is caused by fluid accumulation in the distensible cochlear apex that influences mechanoelectric transducer channels. However, earnest pursuits to quantify the sought-after relationship between hydrops and hearing loss have concluded that the origin of hearing loss measurements is not endolymphatic hydrops. Classic studies could not assess the relationship between low-frequency hearing loss and endolymphatic hydrops, as conventional physiologic measurements (e.g., otoacoustic emissions and the auditory brainstem response) that do not perform well at frequencies below ~1 kHz, which is the frequency region of hearing loss in Ménière’s disease. Our research found that, in the later stages of the condition, the severity of endolymphatic hydrops in the apical cochlear half indeed correlates with the degree of low-frequency hearing loss. This points to endolymphatic hydrops as the origin of the low-frequency hearing loss associated with Ménière’s disease. The clinical and practical utility of this finding is that low-frequency hearing loss may quantify the progression and treatment of endolymphatic hydrops. Some of the current efforts in our laboratory include translating our basic science discoveries to the clinic to provide fresh insight and ultimately help patients that suffer from Ménière’s disease.
- Lichtenhan JT, Lee C, Wenrich KA, Dubaybo F, Wilson US. The auditory nerve overlapped waveform (ANOW) detects small endolymphatic manipulations that may go undetected by conventional measurements. Frontiers of Neuroscience. 2017 18;11:405. PMID: 28769744; PMCID: PMC5513905
- Lee C, Valenzuela CV, Goodman SS, Kallogjeri D, Buchman CA, Lichtenhan JT. Early detection of endolymphatic hydrops using the auditory nerve overlapped waveform (ANOW). Neuroscience. 2020 Jan 15;425:251-266. PubMed PMID: 31809731; PMCID: PMC6935415.
- Valenzuela CV, Lee C, Buchman CA, Lichtenhan JT. A revised surgical approach to induce endolymphatic hydrops in the guinea pig. 2020a. Journal of Visualized Experiments. 4(160). PMID: 32568243.
- Valenzuela CV, Lee C, Mispagel A, Bhattacharyya A, Lefler SM, Payne S, Goodman SS, Ortmann AJ, Buchman CA, Rutherford MA, Lichtenhan JT. Is cochlear synapse loss an origin of low-frequency hearing loss associated with endolymphatic hydrops? 2020b. Hearing Research. PMID: 33125982, PMCID: PMC9058942
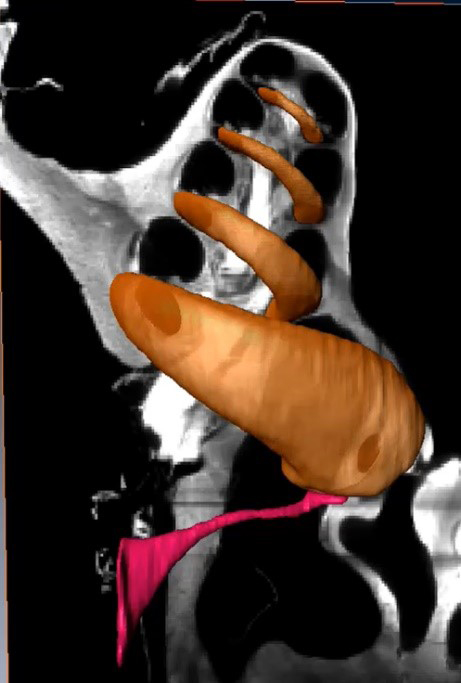
We previously found evidence that stimulus frequency otoacoustic emissions (SFOAEs) originate in the peak region of the traveling wave (Lichtenhan 2012). This finding addressed an ongoing controversy regarding theoretical and empirical evidence on SFOAE generation. The approach was to use intense, very low-frequency tones to attenuate the gain of the cochlear amplifier in the peak region of the traveling wave of a higher frequency probe tone. In the basal half of the cochlea, mechanical amplification is likely most robust at the peak of the traveling wave and gives rise to otoacoustic emissions. Prior to our study, others had used high-frequency sounds to manipulate SFOAE generators, but these sounds created additional distortion sources that complicated measurement and interpretation. While the results of our study were consistent with theoretical models of SFOAE origination, the results were not definitive. We thus developed an apical perfusion technique to provide a coveted approach of manipulating characteristic-frequency regions in a manner that is independent of more basal regions (Lichtenhan et al. 2016). We found that SFOAEs from the basal half of the cochlear spiral originate approximately one-half octave basal to the associated cochlear characteristic frequency place (Goodman et al. 2020). Our current research aims to understand the origin of SFOAEs in the apical half of the cochlea, a region that is affected by endolymphatic hydrops and Ménière’s disease.
- Lichtenhan JT. Effects of low-frequency biasing on otoacoustic and neural measures suggest that stimulus-frequency otoacoustic emissions originate near the peak region of the traveling wave. Journal of the Association for Research in Otolaryngology. 2012. 13(1):17-28. PMID: 22002610; PMCID: PMC3254722.
- Lichtenhan JT, Hartsock JJ, Dornhoffer J, Donovan KM, Salt AN. Drug delivery into the cochlear apex: Improved control to sequentially affect finely spaced regions along the entire length of the cochlear spiral. Journal of Neuroscience Methods. 2016. 273:201-209. PMID: 27506463; PMCID: PMC5075496.
- Goodman SS, Lee C, Guinan JJ Jr., Lichtenhan JT. The spatial origins of cochlear amplification assessed by stimulus-frequency otoacoustic emissions. Biophysical Journal. 2020. 118(5):1183-1195. PMID: 31968228; PMCID: PMC7063421.
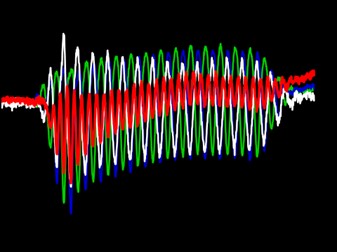
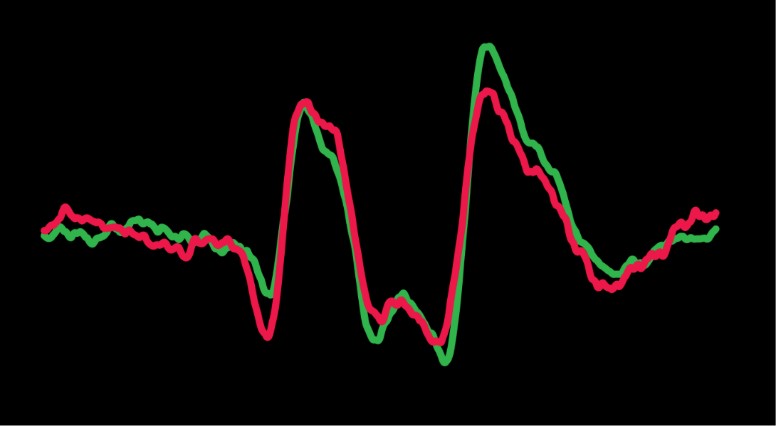
Wave I of the auditory brainstem response, or the auditory-nerve compound action potential (CAP), is used in clinics and laboratories worldwide. The CAP is one component of a response that can be used for a variety of purposes such as identifying and quantifying hearing loss in newborns so that prescriptive targets for hearing aid amplification can be set. The amplitude of the CAP is used to identify ears with cochlear synaptopathy. Despite the wide use of the CAP, no experiment directly identified the spatial origins of the CAP along the cochlear length. We recently used the apical perfusion technique developed in our lab to address this gap in knowledge (Lee et al. 2019). We found that CAPs evoked from low-level tone bursts originate from the cochlear characteristic-frequency place associated with the tone burst, a finding that has never before been empirically demonstrated. As the sound pressure level of lower-frequency tone bursts increases, we found that the spatial origin of the CAP shifts away from the characteristic-frequency place towards the cochlear base by up to two octaves of characteristic frequency place. In contrast, as the stimulus level of high-frequency tone bursts increases, the origin of the CAP shifts toward the cochlear apex. It appears that regardless of the frequency of the evoking tone burst, the spatial origin of the CAP shifts toward the most sensitive region of the audiogram, where thresholds are low and neural density is high. The CAP does not shift continuously toward the cochlear base as level increases as previously thought.
In ears with endolymphatic hydrops, we have discovered that the spatial origin of CAPs shift towards to the cochlear apex, likely because endolymphatic hydrops stiffen the partition (Guinan et al. 2021). This shift is associated with a shift in the cochlear frequency place map that is likely the origin of the diplacusis symptom experienced by most people with Ménière’s disease. Diplacusis is the perception of a tone having different pitches between the left and right ears. Most patients with Ménière’s disease and diplacusis perceive pitch to be lower in the affected ear, which is consistent with our finding that the origin of the CAP shifts towards the most sensitive region. The information gained from our experiments on the spatial origin of the CAP along the cochlea has advanced the interpretation of objective or psychophysical measures of hearing, in both normal ears and ears with overt or hidden hearing loss.
- Lee C, Guinan JJ Jr., Rutherford MA, Kaf WA, Kennedy KM, Buchman CA, Salt AN, Lichtenhan J. Cochlear compound action potentials from high-level tone bursts originate from wide cochlear regions that are offset toward the most sensitive cochlear region. Journal of Neurophysiology. 2019. 121(3):1018-1033. PMID: 30673362; PMCID: PMC6520629.
- Guinan JJ Jr, Lefler SM, Buchman CA, Goodman SS, Lichtenhan JT. Altered mapping of sound frequency to cochlear place in ears with endolymphatic hydrops provide insight into the pitch anomaly of diplacusis. Scientific Reports. 2021. 11(1):10380. PMID: 34001971, PMCID: PMC8128888.
Previous experiments completed in animals found that the strength of the human MOC reflex is greater on auditory-nerve compound action potential (CAP) measurements than on outer-hair-cell-mediated otoacoustic emissions (OAEs). We were the first to translate this research to humans and quantified the strength of the MOC reflex on OAEs and CAPs from individual human ears. The MOC reflex has been studied in humans almost exclusively by measuring changes in otoacoustic emissions. To help understand how the MOC system influences what we hear, it is essential to have measurements on the MOC effect on auditory nerve responses that couples sound to the brain. While our findings are consistent with previous reports from animals, they are interesting because MOC fibers synapse on outer hair cells. Outer hair cells give rise to OAEs, and not the CAP. These results are informative on how the MOC reflex attenuates the process of mechanical amplification, and ultimately auditory nerve excitation, in the human cochlea.
In another study, we found that strong MOC reflexes in children with Autism Spectrum Disorder (ASD) are associated with hyperacusis. In addition, this study determined that hyperacusis is a comorbid condition of ASD and not a necessary, integral part of the abnormal neural processing associated with ASD. Children with Autism often have hyperacusis, which can significantly disrupt their ability to function on a daily basis. For example, intolerance to loud sounds such as industrial-quality toilet flushing at school can lead to physical rage towards teachers. We measured MOC reflexes in three groups of children: children with Autism and hyperacusis, children with Autism but no hyperacusis, and children who are neurotypical. We found that children with Autism and hyperacusis have stronger MOC reflexes than children in the other two groups. These results are consistent with other findings that MOC reflexes are stronger, not weaker, in people with hyperacusis who are otherwise neurotypical (i.e., typical people who do not have Autism [Knudson et al. 2014 J Neurophysiol. 112(12):3197]). These results are the foundation for the development of a novel technique to objectively monitor behavioral treatment of hyperacusis in children with Autism.
- Lichtenhan JT, Wilson US, Hancock KE, Guinan JJ. Medial olivocochlear efferent reflex inhibition of human cochlear nerve responses. Hearing Research. 2016. 333:216-24. PMID: 26364824; PMCID: PMC4788580.
- Wilson, US, Sadler, KM, Hancock, KE, Guinan, JJ Jr., Lichtenhan, JT. Efferent inhibition strength is a physiological correlate of hyperacusis in children with autism spectrum disorder. Journal of Neurophysiology. 2017. 118(2):1164-1172. PMID: 28592687; PMCID: PMC5547266.